Spherulitic lead calcium apatite crystals shed new light on phosphate dosing
Small amounts of phosphate are added to tap waters in the UK to prevent lead leaching from lead water pipes via a process known as phosphate dosing. But scientists are not certain how this works and there may even be more than one mechanism.
Now, a recent discovery from a team of scientists led by the University of Huddersfield, in collaboration with Yorkshire Water, has suggested the presence of certain organic molecules in tap waters could play an important role in the process.
The project has been led by the University’s Dr Jeremy Hopwood, a senior chemistry lecturer who has over a decade of expertise studying and lowering the levels of lead in tap water.
Also included in the research team were the University’s Professor Paul Humphreys, industry experts and affiliates of the University’s School of Applied Sciences, as well as water scientists from Yorkshire Water.
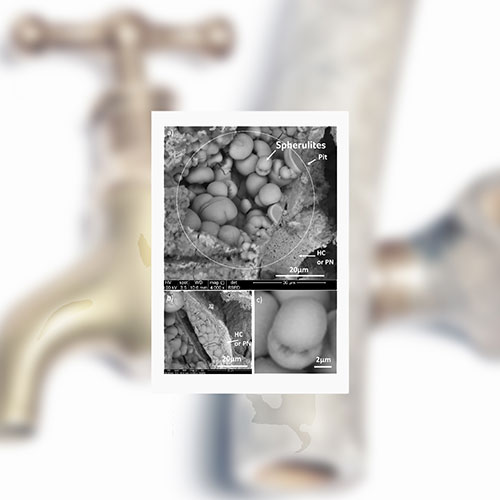
Lead water pipes develop a thin mineral scale as the pipe slowly corrodes and it was whilst investigating this when the team discovered the spherically shaped crystals of lead calcium phosphate. This was unexpected as crystals are usually flat sided or faceted, like diamonds or cubic salt crystals.
Lead calcium phosphate crystals are extremely insoluble in phosphate dosed tap water and so their presence suggests phosphate acts by mineralising the lead, preventing it from leaching into the water. However, the unusual spherical shape of the crystals implies something is interfering with their growth, making them less effective at removing lead.
The team discovered the spherulitic lead calcium apatite could be grown in the laboratory by adding lead carbonate crystals to soft and hard waters containing phosphate, chloride, and citrate ions at pH 5.5, but not when the citrate was absent.
“As the spherulites only formed when citric acid was present,” explained Dr Hopwood, “this suggests that dissolved organic molecules could be a key factor when thinking how and why spherulitic lead calcium apatite forms on lead water pipes,” he said, adding this discovery would be of benefit to water utilities in the UK who needed to gain a better understanding of how phosphate mitigates lead in tap water.
The research also adds to a growing body of work on spherulites which is a hot topic for crystallographers, as these shapes are in other systems.
The University’s long-standing relationship with Yorkshire Water has led to them collaborating on several projects together as part of a continuing strategy to minimise lead solubility and comply with the legal UK limit of 10 micrograms of lead per litre.
This research has been published by the American Chemical Society in the journal ‘Environmental Science and Technology’ titled, ‘Spherulitic Lead Calcium Apatite Minerals in Lead Water Pipes Exposed to Phosphate-Dosed Tap Water’.
Download the journal article for free here: https://pubs.acs.org/doi/10.1021/acs.est.2c04538





















