The importance of understanding protein-protein and protein-ligand interactions in immuno-oncology
[To see figures and tables refer to https://www.cresset-group.com/about/news/immuno-oncology/]
Historical context and significance
Despite cancer being a disease that has been with us for centuries, it has never been as important as it is now as one of the key age limiting factors in modern society. This is because human longevity is steadily increasing, the resulting overall population aging, and cancer increases prevalence with age. Unsurprisingly then, it follows that cancer is a disease that is receiving vast attention from the global medical research community.
Over the years, cancer treatment has developed from being purely surgical, to a barrage of techniques including: radiation treatment, chemotherapy and various targeted therapies to stop or reverse tumor growth. Whilst some of these therapies are relatively blunt instruments (essentially are simply cell killing), one of the key ‘targeted’ mechanisms which has emerged in recent times, is re-setting the signalling responsible for apoptosis (programmed cell death). Ironically, cellular immortality, by subversion of the natural death cycle of cells, is what ultimately leads to death from this disease.
Insights into the unique biology of tumors such as its need for its own vasculature, rapid growth and subsequent hypoxia has stimulated several successful mechanisms for selective anti-tumor therapeutics. Unfortunately, there are a myriad of targets involved in these very complex systems, some involving resistance mechanisms, many proving difficult to drug selectively, safely and efficaciously.
Evading the host immune system and how to reverse it?
Another trick up the sleeve of cancer cells, in addition to evading death, is evading detection by the immune system. Normally, abnormal, aging or infected cells are recognized by the immune system and are destroyed. It has been shown that, in contrast, the microenvironment of the tumor ‘actively’ supresses this immune system response. Pioneering work1, by two independent groups, was the first to identify specific cell surface receptors that supress the activation of T-cells. Two targets: CTLA4 and PD-1 were subsequently successfully exploited with FDA approved monoclonal antibody treatments that block their interactions (table1).
Table1: Anti-cancer biologics targeting checkpoint blockade2
Table 2: Table 1 tumor type Key2
Thus, clearly demonstrating that blocking these receptor interactions can stimulate T-cells and reactivate the immune response towards clearing some cancers. This represents a completely new paradigm3 for cancer treatment ‘checkpoint blockade’ which resulted in the Nobel prize being awarded to the main contributors: James P. Allison and Tasuku Honju in 2018.
Molecular recognition between cells
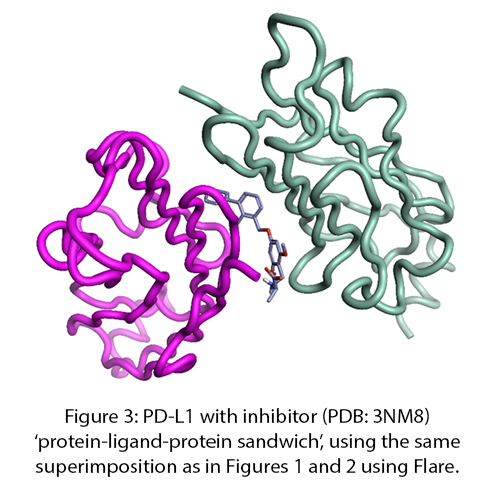
The thing which links both discoveries is the type of molecular recognition operating (figures 1, 2). These are both protein-protein interactions driven through immunoglobulin binding repeats. The relatively flat and extensive surfaces make them less ideal for small molecule intervention and more appropriate to the biologics area – which are themselves also built of immunoglobulin repeats.
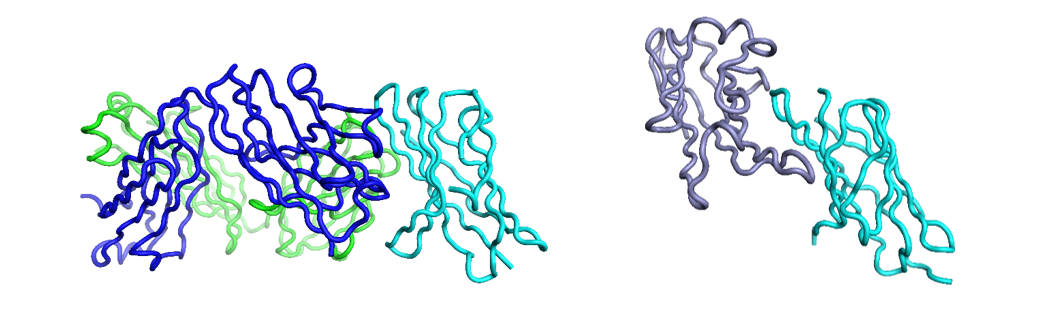
Figure 1: Ipilimumab (blue: heavy, green: light) and CTLA4 (cyan) complex (left, PDB: 5TRU), CTLA4 and B7-2 complex (right, PDB: 1I85) showing how the antibody overlaps, and thus competes with, the interaction of CTLA4 with B7-2. Superimposed for comparison and visualized in Flare™.
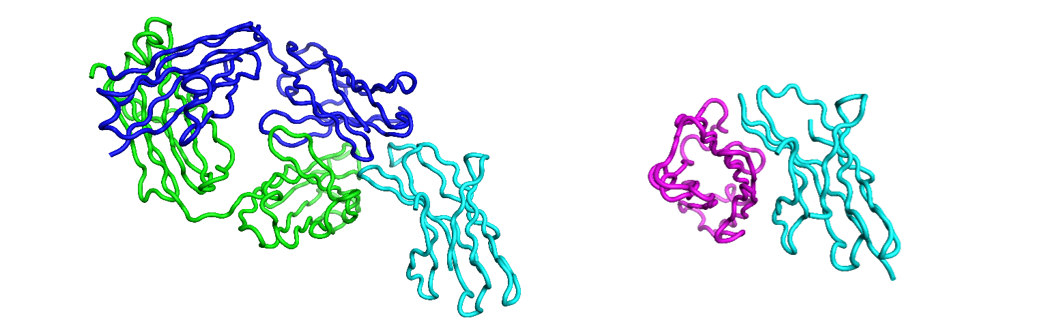
Figure 2: Nivolumab (blue: heavy, green: light) and PD1 (cyan) complex (left, PDB: 5WTN), PD1 and PD-L1 complex (right, PDB: 4ZQK) showing how the antibody overlaps, and thus competes with, the interaction of PD1 with PD-L1, visualized usign Flare.
What can be done in terms of modeling?
A 3D molecular understanding of the binding events being used by these systems is useful:
- In the understanding and manipulating large molecule therapeutics. Cresset Discovery Services has been successful in making accurate predictions about the influence of single point mutations on binding events in protein-protein recognition systems of this type.
- It becomes absolutely critical for the design of ‘potential’ small molecule active therapeutics in this area.
CTLA4 has so far proved to be challenging4, however PD-L1 has been shown to be tractable towards small molecules5,6 and small peptide macrocycles7. One ‘potentially serendipitous’ factor here is the recruitment of two monomers of the PD-L1 unit - to create a ‘protein-ligand-protein sandwich’ interface - that prevents PD1 binding (Figure 3).
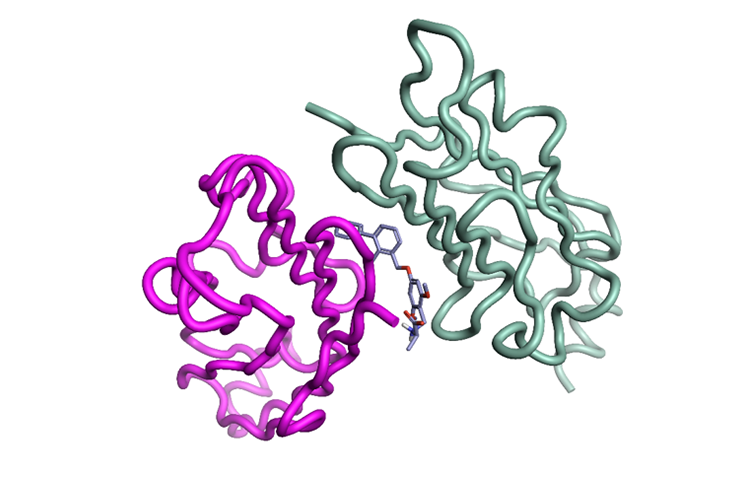
Figure 3: PD-L1 with inhibitor (PDB: 3NM8) ‘protein-ligand-protein sandwich’, using the same superimposition as in Figures 1 and 2 using Flare.
Conclusion
The accuracy of analyzing protein-protein and protein-ligand interactions are fundamental to understanding recognition events in complex binding systems. Whilst shape and H-bonding are important for simple enzymatic systems, electrostatics and hydrophobics (solvation/de-solvation) are far more important for binding.
Apply Cresset Discovery Services expertise to your project
Contact us for a free confidential discussion to see how our expert computational chemists can help advance your projects.
References
- The Nobel Prize in Physiology or Medicine 2018. NobelPrize.org. Nobel Media AB 2019. Fri. 19 Jul 2019.
- Lessons in fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Wei S. C.; Duffy C. R., and Allison J.P.; Cancer Discovery; 2018 8(9); 1–18.
- Immuno-Oncology: Emerging Targets and Combination Therapies, Marshall H. T. and Djamgoz M. B. A.; Frontiers in Oncology 2018), 8 Article 315
- Small-Molecule Targets in Tumor Immunotherapy, Natural Products and Bioprospecting, Zhu, H., Li Y. 2018; 8:297–301
- Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Death-Ligand 1 (PD-1/PD-L1) Interaction via Transiently Induced Protein States and Dimerization of PD-L1, Guzik K., Zak K. M., Grudnik P., Magiera K., Musielak B., Törner R., Skalniak L., Dömling A., Dubin G., Holak T.A; J. Med. Chem. 2017 60 13 5857-5867.
- Fragment-based screening of programmed death ligand 1 (PD-L1). Perry E., Mills J.J., Zhao B., Wang F., Sun Q., Christov P.P., Tarr J.C., Rietz T.A., Olejniczak E.T., Lee T, Fesik S. Bioorg Med Chem Lett. 2019 Mar 15; 29(6):786-790.
- Bioactive Macrocyclic Inhibitors of the PD-1/PD-L1 Immune Checkpoint, Magiera-Mularz K., Skalniak L., Zak K. M., Musielak B., Rudzinska-Szostak E., Berlicki L., Grudnik P., Sala D., Zarganes-Tzitzikas T., Shaabani S., Magiera K., Dömling A., Dubin G., Holak T.A; Angew. Chem. 2017, 129, 13920– 13923





















