Mpox: Emerging Challenges and Pharmaceutical Innovations in the Face of a Global Health Crisis
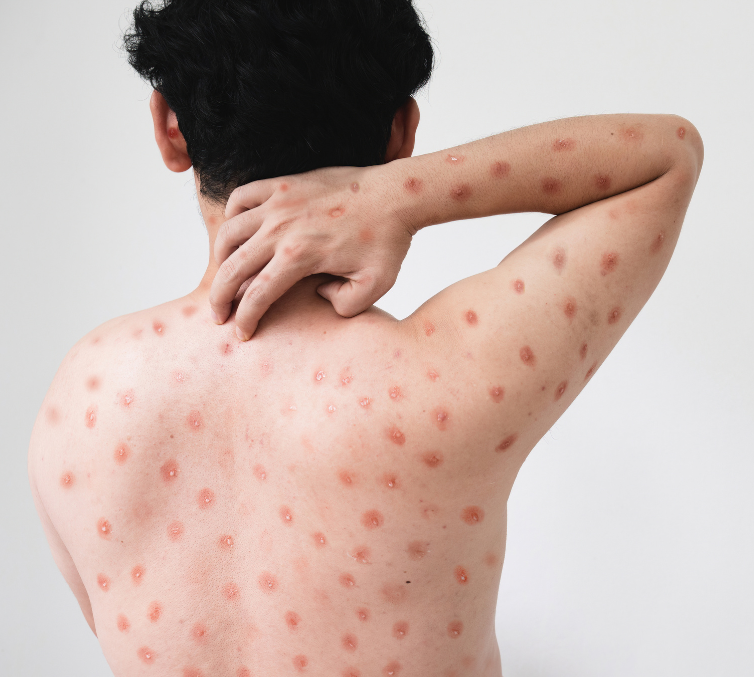
Mpox, previously known as monkeypox, has been an endemic disease in a number of African countries for decades, but it gained significant attention during the first global outbreak in 2022. Although this outbreak was brought under control within a year, the emergence of new viral clades has complicated the situation. Notably, a rapid spread of the virus called Clade Ib has been reported in the Democratic Republic of the Congo, with recent cases also detected in Sweden and Thailand. Other clades, such as Clade Ia and Clade II, continue to circulate in various African nations, leading to a renewed declaration of public health emergency of international concern (PHEIC) by the WHO in August 2024. As of June 2024, nearly 100,000 cases and over 200 deaths have been documented across 116 countries.[1]
What is mpox?
Mpox is a viral illness caused by the monkeypox virus, which belongs to the Orthopoxvirus genus in the Poxviridae family. The Poxviridae family also includes other viruses such as variola, cowpox, and vaccinia. Mpox is classified into two main clades: clade I, which has subclades Ia and Ib, and clade II, which includes subclades IIa and IIb.
The common symptoms of mpox include a skin rash or mucosal lesions that can persist for 2 to 4 weeks, often accompanied by fever, headache, muscle aches, back pain, fatigue, and swollen lymph nodes. Transmission of mpox occurs through close contact with an infected individual, exposure to contaminated materials (such as clothing or linens), or interaction with infected animals, which may involve bites or scratches.
Treatment for mpox primarily focuses on supportive care to alleviate symptoms such as pain and fever. This includes careful attention to nutrition, hydration, skin care, and the prevention of secondary infections and co-infections. Diagnosing mpox can be challenging, as its symptoms may resemble those of other infections. [2]
Treatments for mpox
While some antiviral medications have received emergency use authorization in certain countries and are currently under evaluation in clinical trials, there is, as of now, no proven effective antiviral treatment specifically for mpox.
A vaccine that has been used in the United Kingdom to treat mpox is the Modified Vaccinia Ankara (MVA) vaccine (under the brand name Imvanex). The MVA vaccine is a weakened form of the vaccinia virus, a virus closely related to the smallpox and mpox, but does not cause the disease in humans.
The antiviral agents cidofovir, brincidofovir, and tecovirimat are also known to have activity against mpox. Cidofovir and brincidofovir (a prodrug of cidofovir) are inhibitors of DNA replication with activity against multiple families of double-stranded DNA viruses. Tecovirimat has more specific activity against orthopoxviruses and blocks cellular transmission of the virus.
Tecovirimat: a patent filing strategy case study
Tecovirimat (sold under the brand name Tpoxx, among others), is an inhibitor of the orthopoxvirus VP37 envelope wrapping protein.
The first synthesis of tecovirimat was published in 2004 by SIGA Technologies Inc. (see WO2004/112718). Since then SIGA Technologies Inc. has filed several patent applications (many of which are now granted) relating to various aspects of the tecovirimat technology, including: polymorphic forms of tecovirimat (see, for example, US9339466B2), pharmaceutical compositions containing tecovirimat (see, for example, US8124643B2), methods of treating orthopoxvirus infection by administering a therapeutically effective amount of tecovirimat (see, for example, US7737168B2), formulations containing tecovirimat (see, for example, US10576165B2), and processes of preparing tecovirimat (see, for example, US9045418B2). The most recent patent application filed by SIGA Technologies Inc. on the tecovirimat technology was filed in 2017 and relates to a dry suspension of tecovirimat monohydrate and simethicone (now granted in the US and pending in Europe) (see, for example, WO2017/142910).
SIGA Technologies Inc. patent filing strategy serves as an interesting case study for extending a drug’s commercial lifecycle. The patent filing in 2017 has the potential to extend patent protection for SIGA Technologies Inc.’s tecovirimat technology until 2037, which is about 33 years from the first filing in 2004.
Patent filings as a means for extending a drugs commercial lifecycle
In the highly competitive pharmaceutical industry, extending the commercial lifecycle of a drug is crucial for maximizing return on investment and ensuring sustained market presence. Patent filing strategies play a pivotal role in this process, as they provide the legal framework necessary to protect innovations and prevent market entry by generic competitors. By strategically filing patents at various stages of a drug's development and commercialization, companies can secure exclusive rights to prolong their market exclusivity. Pharmaceutical companies can extend a drug’s commercial lifecycle using patents through several strategic approaches. For example, companies may file further patent applications to:
- New formulations of existing drugs. Examples of new formulations include extended-release or controlled release formulations of the drug, or a new delivery system that improves the drug’s efficacy or a patient’s compliance.
- New therapeutic uses for an existing drug. In Europe, second or further medical uses are patentable provided the use is novel and inventive over what is already known. However, this principle applies only to substances and compositions, and cannot be extended to other products. In Europe, it is also possible to obtain a patent to new dosage regimens, treatment protocols, or patient populations.
- Combination therapies. A combination of two or more active ingredients may create a new composition of matter. Such a combination is patentable if it has not been disclosed previously, and it provides an unexpected technical effect. In Europe, the unexpected technical effect must be a technical effect beyond the mere aggregation of the known effects of the individual components.
- Biologics and biosimilars. Biologics, which are large, complex molecules derived from living organisms, may be patented if they meet the standard patentability criteria. Biosimilars are products that are highly similar to an already approved reference biologic and must usually have no clinically meaningful differences over the reference biologic. However, manufacturing processes can often be patented covering the production of both the reference biologic and biosimilar to extend patent term.
- Secondary patents. Filing secondary patents on improvements or modifications to the original drug can provide additional layers of protection. These may include patents on new manufacturing processes, polymorphs, or new delivery mechanisms.
Other ways of extending the term of protection
It is also possible to extend the term of a medicinal patent using supplementary protection certificates (in many European countries) or Patent Term Extensions (PTEs) (in the United States of America). For example, SPCs are a form of intellectual property right designed to extend the duration of patent protection for specific pharmaceutical products that have received authorization from regulatory authorities. SPCs are intended to compensate for the loss of patent protection that pharmaceutical products experience due to the extensive testing and clinical trials mandated before they can obtain regulatory marketing approval. SPCs can prolong the patent term of a pharmaceutical product by as much as five years, with the potential for an additional six-month extension under certain conditions.
In Europe, the protection period for drugs can be further enhanced through regulatory data exclusivity and drug market exclusivity (the so-called “8 + 2 formula”). When a drug is the first of its kind to receive approval from the European Commission (or an individual member state) and is supported by a full marketing authorization application dossier, it is entitled in parallel to an eight-year regulatory data protection period and a ten-year market protection period. During the eight-year regulatory data protection period, companies seeking to develop generic, hybrid, or biosimilar versions of the drug are prohibited from referencing the original drug's marketing authorization application dossier to obtain their own marketing authorization. During the ten-year market protection period (e.g., during the additional two years following expiry of regulatory data protection), they cannot launch their products in the market.
Additionally, a one-year extension of market exclusivity may be granted for new therapeutic indications that demonstrate a significant clinical benefit over existing therapies. Similarly, a one-year extension of regulatory data exclusivity can be awarded for a new therapeutic indication of a well-established substance, contingent upon the completion of substantial pre-clinical or clinical studies related to the new indication. A one-year extension of regulatory data exclusivity can also be awarded in cases where there is a change in the classification of a medicinal product based on significant pre-clinical tests or clinical trials.
Furthermore, orphan medicines benefit from additional market exclusivity incentives, which can provide an extra two years of market exclusivity (on top of the standard ten years) upon the successful completion of a paediatric investigation plan.
Final thoughts
The resurgence of mpox as a significant public health issue highlights the intricate interplay between infectious disease management and pharmaceutical innovation. The emergence of new viral clades and the subsequent global health concerns have highlighted the necessity for innovative treatments and vaccines. The case of tecovirimat serves as a pertinent example of how strategic patent filings can extend the commercial lifecycle of a drug, ensuring its availability and accessibility in the market. By securing patents on various aspects of drug development – from formulations to new therapeutic uses – pharmaceutical companies can protect their innovations and incentivize further research. This approach not only benefits the companies involved but also contributes to the broader goal of enhancing public health responses to infectious diseases like mpox.
For more information about IP strategy and a compliementary consultation with a patent attorney, visit secerna.com.
[1] https://www.who.int/publications/m/item/mpox-global-strategic-preparedness-and-response-plan





















