Peptide Synthesis Optimisation at Ab Biotechnology
Ab Biotechnology were approached by a client to produce a 29-mer peptide of interest (Figure 1). The peptide had been produced in the past and basic protocols existed for technical transfer to Ab Biotechnology.
Initial review suggested some areas for concern; synthesis would be complicated by several arginine residues and hydrophobic regions. Analysis of historic quality data also indicated the synthesis was partly truncated and the peptide was prone to stability degradation over time.
The approach agreed with the client was to manufacture a proof-of-concept batch as per historic protocols, then determine if the method was suitable for full scale manufacture.
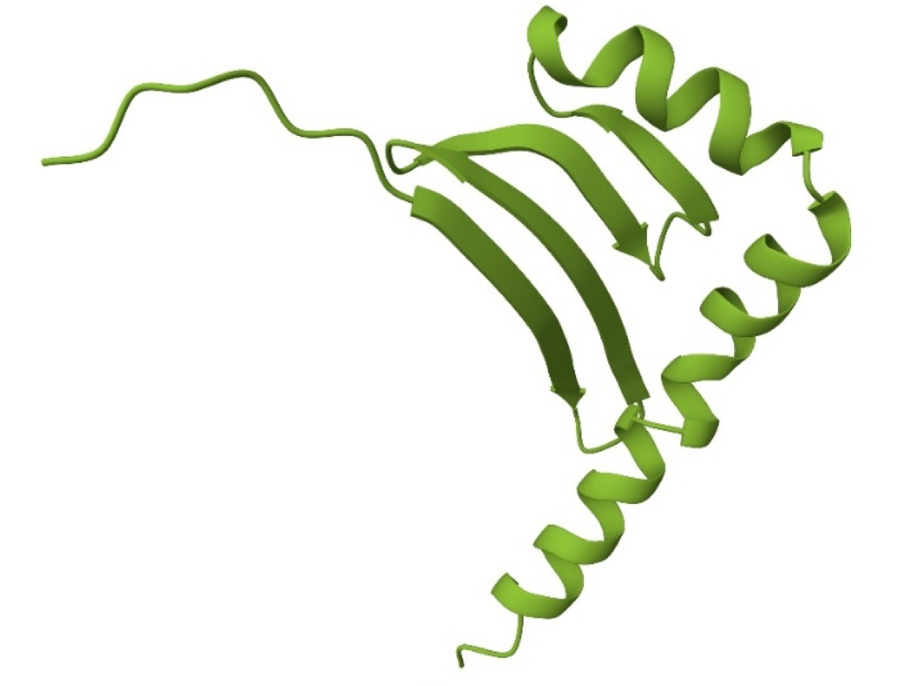
Figure 1. Cartoon representation of the peptide of interest.
The first batch was produced on a pre-loaded Wang resin at 0.1 mmol scale using our standard protocols (Table 1, entry 1). This process yielded the crude peptide with moderate purity (50%, Figure 2, top). Subsequent purification by preparative RP-HPLC resulted in approximately 25% recovery of pure peptide (purity > 95%, Figure 2, bottom).
Although all quality attributes were met and desired level of peptide delivered to the client, the recoveries were lower than expected. We then had to ask ourselves some key questions:
- Were the recoveries acceptable? What were the cost implications for lower than expected yields in terms of number of reaction vessels and corresponding reagent costs and time required in the laboratory?
- Would it be more effective to go back and optimise the synthesis and what were the cost savings in consumables and time implications for delivery?
Although there was no guarantee of success, the consensus was to go back to the drawing board; our Process Development team were confident they could do a better job!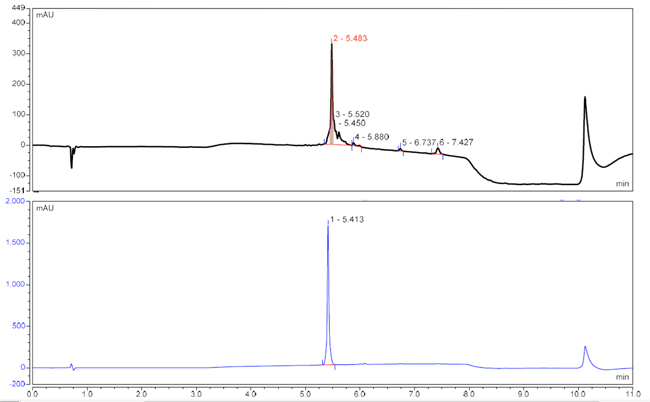
Figure 2. Top: analytical RP-HPLC of crude target peptide (unoptimised synthesis), tR 5.483 min.; bottom: analytical RP-HPLC of purified target peptide, tR 5.413 min.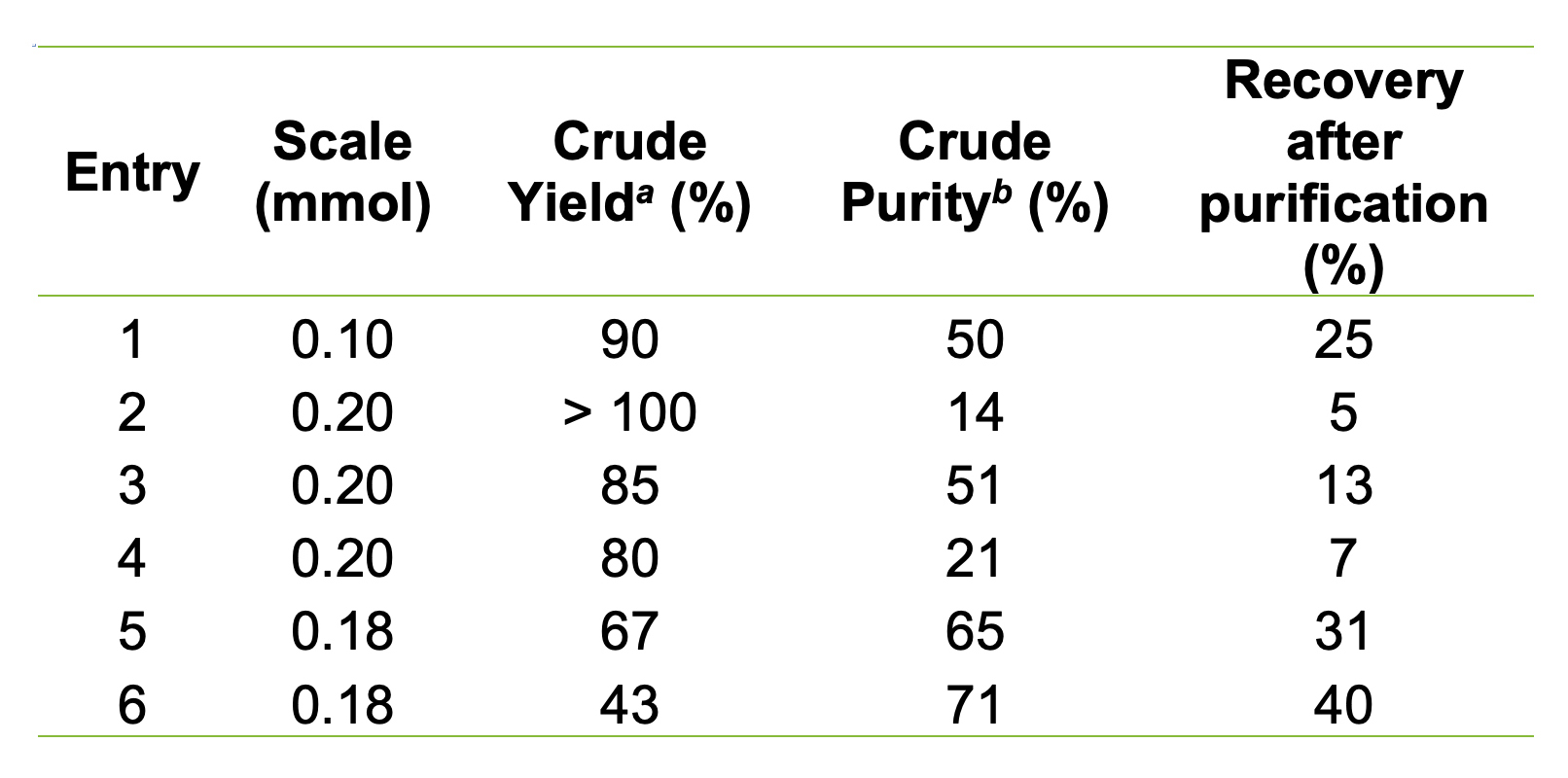
Table 1. Summary of process optimisation results. aCrude yield of lyophilised product before purification, compared with theoretical crude yield. bCrude purity measured by RP-HPLC.
Initial efforts focused on analysis by mass spectrometry of the crude peptide, which allowed identification of capped truncated peptide by-products. Attempts to suppress these by-products by adjusting coupling cycles, temperature, and reagent concentrations yielded minimal improvements - and in some cases, even reduced crude purity (Table 1, entries 3-4). These findings suggested that secondary interactions within the growing peptide chain and with the resin were compromising effective amino acid coupling, necessitating substantial changes to the synthesis protocol.
Subsequent research identified a more hydrophilic resin as a suitable alternative, which provided reduced resin-peptide hydrophobic interactions. Additionally, a pseudoproline dipeptide was introduced into the peptide sequence at a strategic position, to disrupt secondary interactions. Alternative carbodiimide-based and uronium-based coupling reagents were also screened.
Under the optimised conditions (Table 1, entries 5-6), the target peptide was successfully synthesised at 0.18 mmol scale, achieving 65-71% crude purity (Figure 3) and 31-40% recovery after preparative RP-HPLC purification (purity > 95%).
Synthesis is now complete, and three key project deliverables were met:
• Batch size, desired peptide amount delivered (they even got some extra!)
• All quality attributes have been met in terms of purity, mass, identity and so far, in-house stability indicates that peptide is robust
• Time, having evaluated the initial concerns about the peptide, providing a realistic timeline for delivery was key to meeting clients’ expectations.
Although this project was a success, it is important to recognise that optimisation might not be the right decision for every project. The key to any project success is having the right people and the right environment with continual evaluation of project viability in terms of time/cost and quality.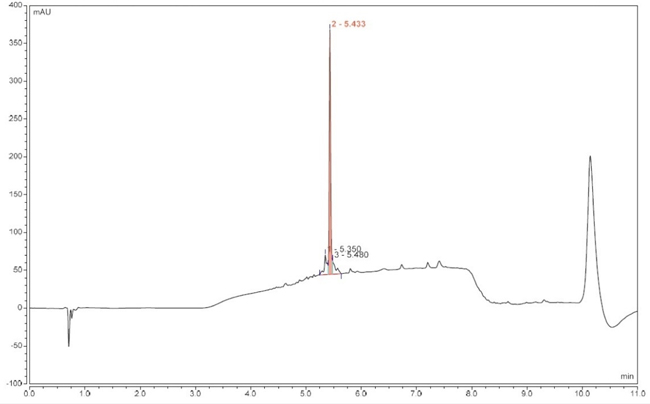
Figure 3. Analytical RP-HPLC of crude target peptide (optimised synthesis), tR 5.433 min.
Please get in contact if you would like to see how we can support your projects.
enquiries@abbiotechnology.com

