Paxman cancer treatment KTP earns Outstanding rating from UKRI
A partnership between the University of Huddersfield and local medical technology company Paxman that could benefit cancer patients undergoing chemotherapy has earned an Outstanding grade from UK Research and Innovation (UKRI).
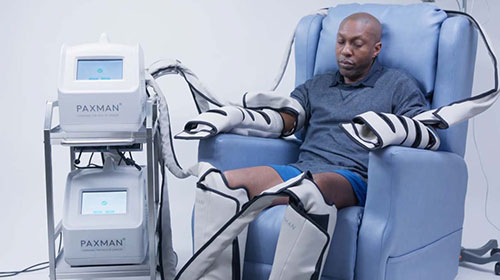
The award is the culmination of a two-year Knowledge Transfer Partnership (KTP) that designed and developed a world-first portable medical cryocompression device for the unmet clinical need of chemotherapy-induced peripheral neuropathy (CIPN).
Building upon a collaboration between the University and Paxman that began in 2012, the KTP saw the design and development of cooling wraps, worn on limbs, that can reduce the severity of CIPN while a patient undergoes chemotherapy.
CIPN is a common, dose-limiting side-effect experienced by 30 to 40 per cent of patients receiving chemotherapy for cancer, with no effective prevention and treatment strategies previously in existence. It causes pain or sensitivity in the hands and feet and can lead to delays, or even the end of treatment.
Company supervisor Jonathan Binder, who is also a PhD student at the University of Huddersfield, was a previous KTP associate. Gayathri Kopattil, a BA (Hons) Product Design graduate from the University, has undertaken an MA in Research in Art and Design and is the KTP Associate for this project.
The academic lead, Dr Ertu Unver, previously worked with the Academic supervisor Dr Omar Huerta who recently moved to the University of Leeds during the second phase of the project.
Dr Unver views the collaboration with Paxman as an exceptional opportunity for the academic team delivering Art and Humanities courses. The team has garnered numerous awards, published journal articles, presented at conferences, and takes pride in their creation of the award-winning medical device, which is subject to numerous global regulatory compliance approvals including clearance by the US FDA, PMDA in Japan and the EU Medical Device Regulation (MDR) certification.
The Paxman Scalp Cooling System is used by cancer patients in over 60 countries, to manage a side-effect widely recognised as one of the most traumatic associated with cancer treatment: chemotherapy-induced alopecia (CIA).
Research had shown that cooling the limbs during chemotherapy could reduce discomfort for patients, but ice packs or frozen gloves provided unreliable cooling. The research stated there was a need for a medical device to be developed which patients could use in a chemotherapy suite and that would deliver stable cooling, tolerable over the entire duration of the chemotherapy.
Richard Paxman, CEO of Paxman, said: “During the past several years, our collaborative work with the University of Huddersfield has had a significant impact on our business and ultimately, the patients benefitting from treatment to alleviate chemotherapy-induced alopecia and in the future chemotherapy-induced peripheral neuropathy.
“The most recent development in the area of CIPN has been a collaboration with the National University of Singapore (NUS) and their affiliated cancer centre. We are currently conducting ongoing clinical trials in both the USA and Singapore."
Huddersfield-based Paxman has an established leadership position in the Scalp Cooling sector and manufacture their medical device at their Fenay Bridge premises. In 2019 they backed the establishment of the Scalp Cooling Research Centre at the University.
This is the third KTP between the University and Paxman and the third to be graded ‘Outstanding’, the highest possible rating, by Innovate UK.
The Paxman Scalp Cooling Research and Innovation Centre team is dedicated to exploring the development of innovative, individually tailored caps and is currently focused on pioneering new cooling technologies for CIPN.

























